Medical Device Braiding
Galway Biomedical has invested in advanced braiding technology to address the changing needs of modern medical devices. Inhouse innovative equipment incorporates sophisticated tension control and fully automated PLC software which facilitates versatile, and extremely low-profile braid designs. With specially designed contact surfaces, Galway Biomedical assures the secure handling of even the most fragile biomaterials, placing the company at the leading edge of medical device braiding.
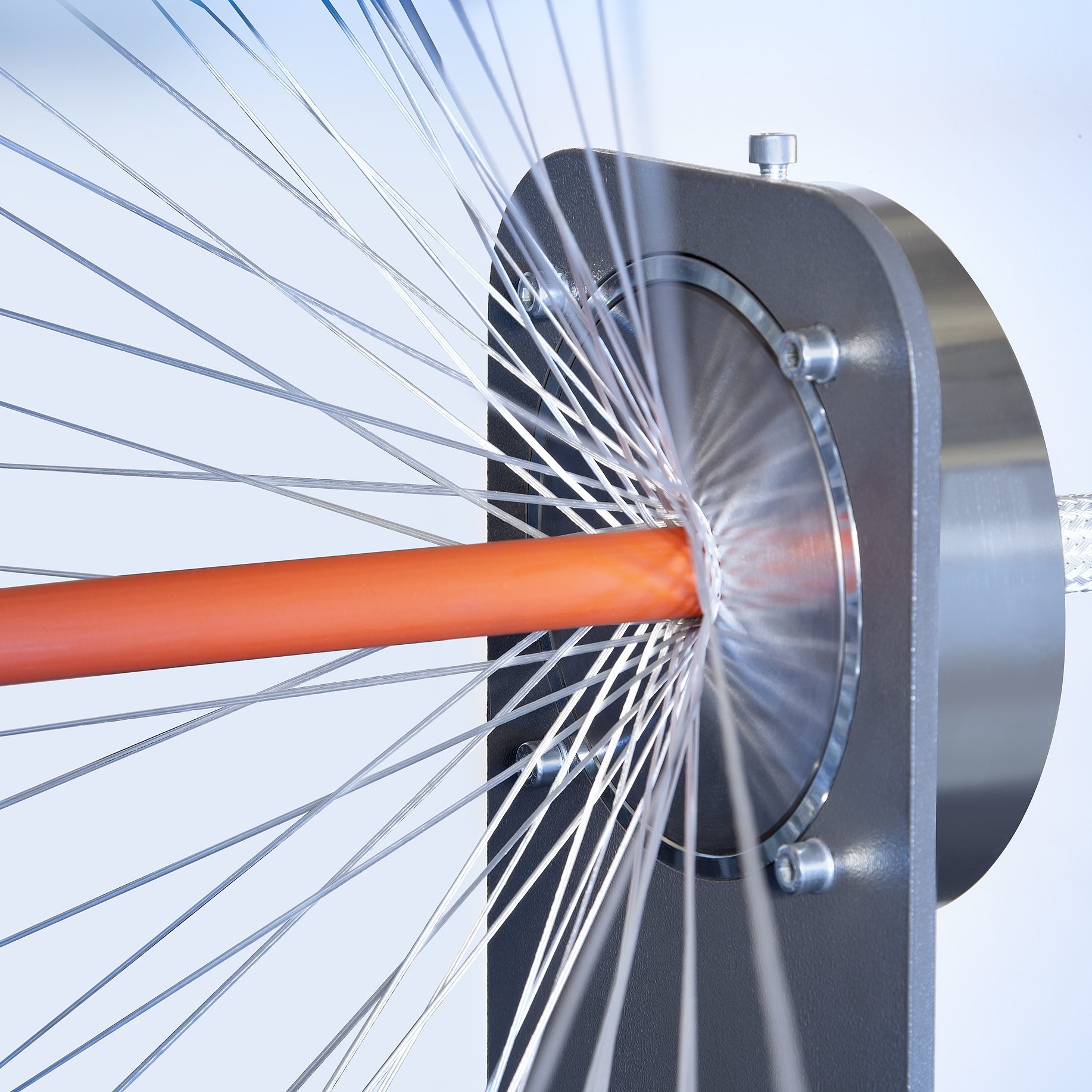
Braiding Capabilities
- Any braid construction from 3-96 ends
- Wire diameters ranging from 12.7µm to 400µm
- Low Dtex/Denier Fibre Processing from 9 Denier/10Dtex
- Continuous Reel-to-Reel and Over Mandrel Braiding
- Complex shape forming of braids
- Controlled braid angles for tailored radial and torsional strength
- Closed end braids (atraumatic/castellated)


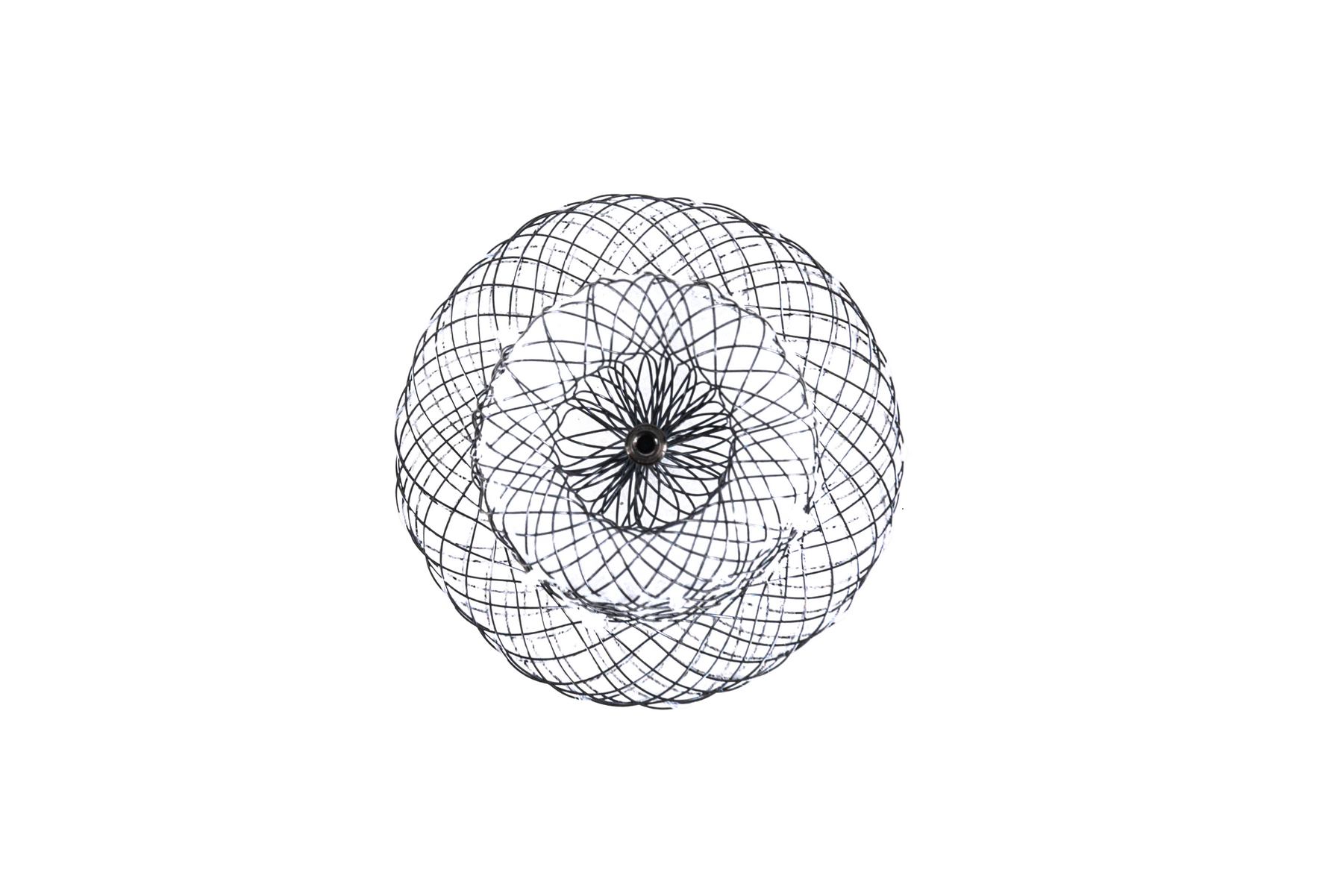

Biomaterials
Galway Biomedical includes both metal and polymer biomaterials in its complex & advanced braiding capabilities, leveraging an international supply network to source a wide range of implantable grade biomaterials.

Wire Braiding
The choice of material in a braided device significantly influences its mechanical properties. For medical braiding applications Galway Biomedical utilizes a variety of round and flat wires, including:
- Nitinol (Grades: #1, #2, #4, #9)
- DFT Composites
- Stainless Steel
- Tungsten
- Platinum
Polymer Braiding
For medical braiding applications Galway Biomedical utilizes a variety of non-resorbable biocompatible materials, in both mono- and multi-filament configurations, including:
- Ultra-High Molecular Weight Polyethylene (UHMWPE)
- Polyester (PET)
- Nylon
- Polypropylene (PP)
- Liquid Crystal Polymer (LCP)
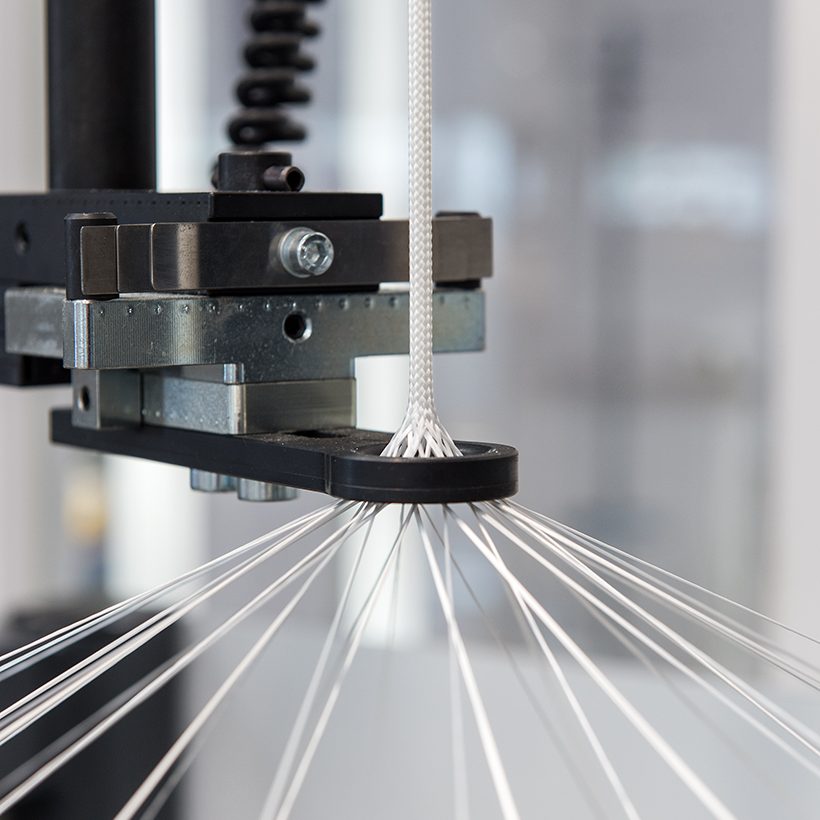
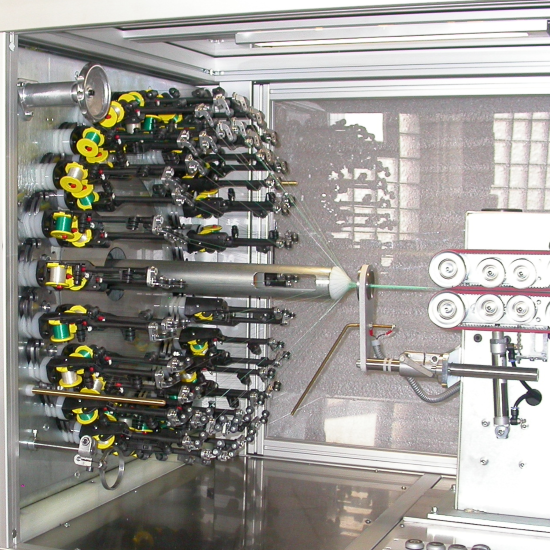
Resorbable Polymer Braiding
Galway Biomedical’s expertise encompasses the preparation, processing and post-processing of resorbable materials. Some of the common resorbable materials utilised include:
- Polydioxanone (PDO)
- Polyglycolic Acid (PGA)
- Polylactic Acid (PLA, PLLA)
- Polycaprolactone (PCL)
- Polylactide/Glycolide Copolymers (PGLA)
- Magnesium Wire
Bespoke Material Braiding
Galway Biomedical offers braiding for bespoke, patented materials owned by clients. If you don’t see your desired material listed above, please reach out for an assessment of your material and its compatibility with Galway Biomedical’s production equipment.
Finishing
Spin finish enhances the processability of biomaterial fibres leading to improved filament cohesion and reduced friction.
Shape setting allows for components to be moulded into distinct and complex configurations. The device can then be crimped for low profile delivery, expanding into the preformed shape upon deployment.
Packaging is offered in line with client’s requirements in an ISO Class 8 cleanroom. This includes bulk packing, label production and full production traceability.
Following early stage prototype feasibility, process optimisation stages and manufacturing transfer, Galway Biomedical offers medical device volume manufacturing.
Work with Us
Galway Biomedical’s mission is to support clients seeking biomaterial based, low profile, complex and advanced braiding solutions.
Reach out to Galway Biomedical today to accelerate your project goals.
